Bimzelx® (bimekizumab-bkzx) from UCB is now FDA approved for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It is the first and only approved psoriasis treatment designed to selectively inhibit both interleukin 17A (IL-17A) and interleukin 17F (IL-17F).
Approval of Bimzelx was supported by data from three Phase 3, multicenter, randomized, placebo and/or active comparator-controlled trials (BE READY, BE VIVID, and BE SURE), which evaluated the efficacy and safety of BIMZELX in 1,480 adults with moderate-to-severe plaque psoriasis.
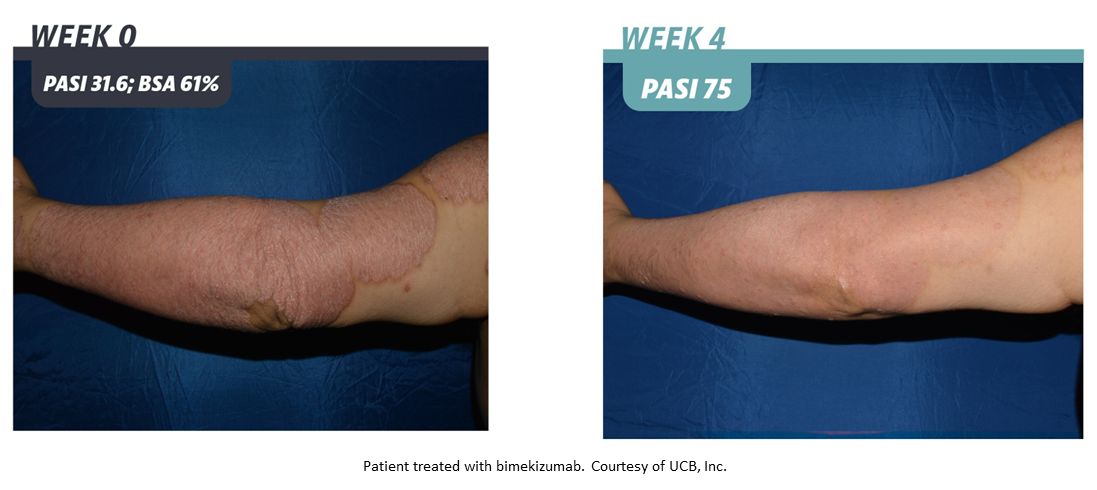
In trials, patients treated with Bimzelx achieved superior levels of skin clearance at week 16, compared to those who received ustekinumab (ranked secondary endpoint, BE VIVID; p<0.0001), placebo (co-primary endpoint, BE READY and BE VIVID; p<0.0001) and adalimumab (co-primary endpoint, BE SURE; p<0.001), as measured by at least a 90 percent improvement in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1).
“We have been eagerly awaiting bimekizumab, the first IL-17A and IL-17F inhibitor, to be approved in the U.S. for the treatment of adults with moderate-to-severe plaque psoriasis. In Phase 3/3b trials, bimekizumab achieved superior levels of skin clearance at week 16 compared to placebo and three existing biologics for psoriasis, with responses being rapid and lasting up to a year. Long-term data have also shown that the majority of patients maintained high levels of clinical response through three years,” said Mark Lebwohl, MD, bimekizumab investigator, Dean for Clinical Therapeutics, Icahn School of Medicine at Mount Sinai, and Chairman Emeritus, Kimberly and Eric J. Waldman Department of Dermatology.
Bimzelx is indicated as a dose of 320mg (given as two subcutaneous injections of 160 mg each) at Weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥120 kg, a dose of 320 mg every 4 weeks after week 16 may be considered. Bimzelx may be administered by a healthcare professional, or a patient may self-inject after proper training. It is available as an autoinjector and a pre-filled syringe.
The most common adverse reactions (≥ 1%) associated with Bimzelx are upper respiratory infections, oral candidiasis, headache, injection site reactions, tinea infections, gastroenteritis, Herpes Simplex Infections, acne, folliculitis, other Candida infections, and fatigue.