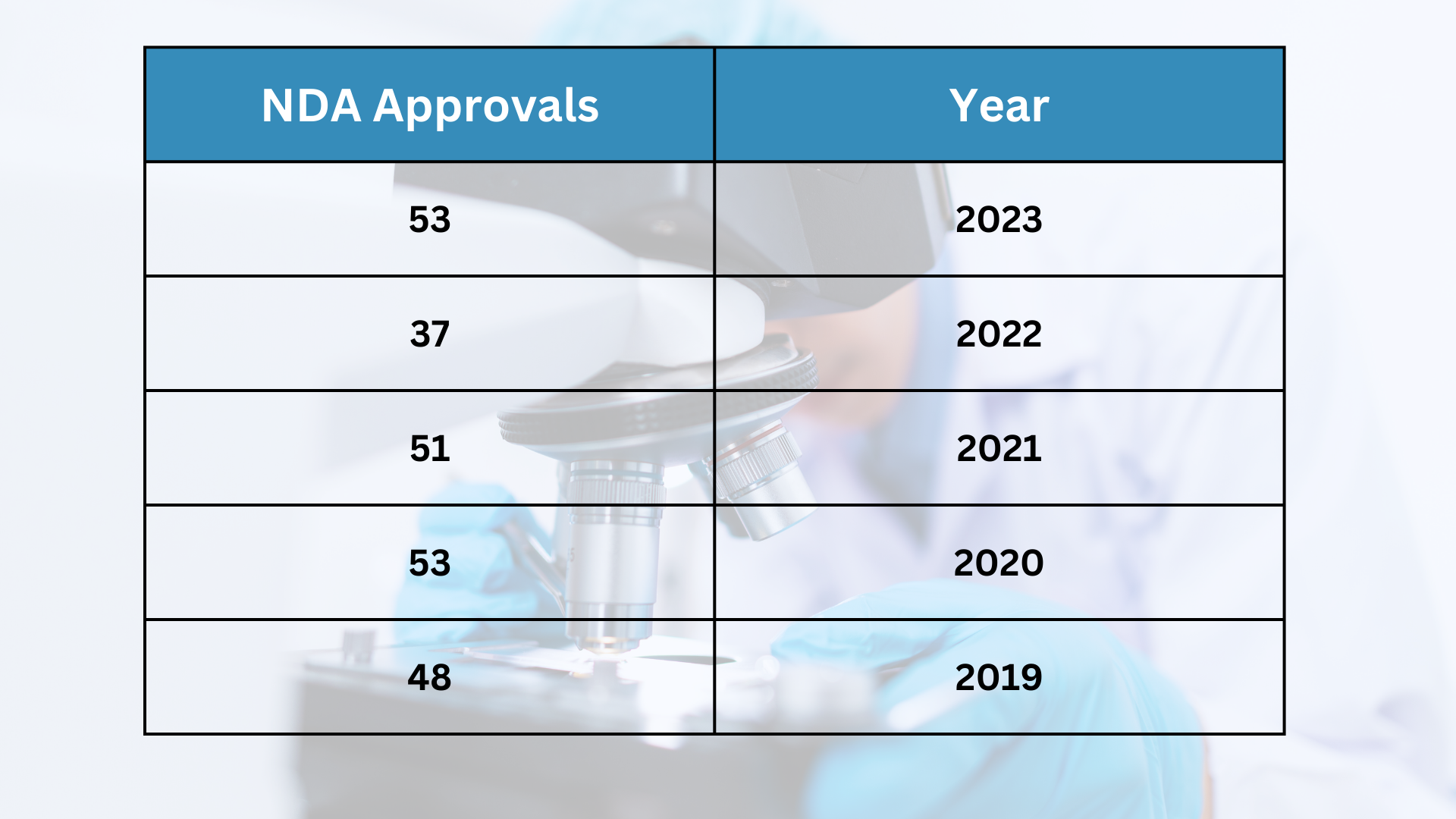
— UPDATE: NDA Approvals for 2023 now stand at 54, with a fourth dermatologic indication approved! Filsuvez® (birch triterpenes) topical gel from Chiesi Global Rare Diseases has received FDA approval for the treatment of partial thickness wounds in patients 6 months and older with Junctional Epidermolysis Bullosa (JEB) and Dystrophic Epidermolysis Bullosa (DEB). Read more here. —
The rate of new drug approvals in the US has fluctuated over the past few years, from just 37 in 2022 to 53 in 2020. FDA approvals of New Drug Applications (NDA) for 2023 currently stand at this pandemic-era level.
Three new drug approvals came in the dermatology space in 2023.
Most recently, Bimzelx® (bimekizumab-bkzx) from UCB, Inc. received approval for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It is the first and only approved psoriasis treatment designed to selectively inhibit both interleukin 17A (IL-17A) and interleukin 17F (IL-17F). This drug gives psoriasis patients the chance to have skin that is clear!
Approval of Bimzelx was supported by data from, which evaluated the efficacy and safety of BIMZELX in 1,480 adults with moderate-to-severe plaque psoriasis.
In three Phase 3, multicenter, randomized, placebo and/or active comparator-controlled trials (BE READY, BE VIVID, and BE SURE), patients treated with Bimzelx achieved superior levels of skin clearance at week 16, compared to those who received ustekinumab (ranked secondary endpoint, BE VIVID; p<0.0001), placebo (co-primary endpoint, BE READY and BE VIVID; p<0.0001) and adalimumab (co-primary endpoint, BE SURE; p<0.001), as measured by at least a 90 percent improvement in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1).
Bimzelx is indicated at a dose of 320mg (given as two subcutaneous injections of 160 mg each) at Weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥120 kg, a dose of 320 mg every 4 weeks after week 16 may be considered. Bimzelx may be administered by a healthcare professional, or a patient may self-inject after proper training. It is available as an autoinjector and a pre-filled syringe.
The most common adverse reactions (≥ 1%) associated with Bimzelx are upper respiratory infections, oral candidiasis, headache, injection site reactions, tinea infections, gastroenteritis, Herpes Simplex Infections, acne, folliculitis, other Candida infections, and fatigue.
Also approved this year was Litfulo (ritlecitinib)
Pfizer’s Litfulo™ (ritlecitinib) 50mg is approved as a once-daily oral treatment for individuals 12 years of age and older with severe alopecia areata. It is the first and only treatment approved by the FDA for adolescents (age 12+) with severe alopecia areata.
Litfulo is an inhibitor of Janus kinase 3 (JAK3) and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) family of kinases.
FDA approval was based on results of the ALLEGRO Phase 2b/3 trial, which enrolled 718 patients with 50% or more scalp hair loss as measured by the Severity of Alopecia Tool (SALT). In this pivotal study, 23% of patients treated with Litfulo 50mg had 80% or more scalp hair coverage (SALT≤20) after six months compared to 1.6% with placebo. The efficacy and safety of Litfulo were consistent between adolescents (12 through 17 years of age) and adults (18 years of age and older). The most common adverse events (AEs) reported in at least 4% of patients with Litfulo include headache (10.8%), diarrhea (10%), acne (6.2%), rash (5.4%), and urticaria (4.6%). Results from the ALLEGRO Phase 2b/3 study were published in The Lancet .
The BLA for Incyte’s Zynyz™ (retifanlimab-dlwr), a humanized monoclonal antibody targeting programmed death receptor-1 (PD-1), was approved under accelerated review for the treatment of adults with metastatic or recurrent locally advanced Merkel cell carcinoma (MCC).
FDA approval was based on data from the POD1UM-201 trial, an open-label, multiregional, single-arm study that evaluated Zynyz in adults with metastatic or recurrent locally advanced MCC who had not received prior systemic therapy for their advanced disease. Among chemotherapy-naïve patients (n=65), Zynyz monotherapy resulted in an objective response rate (ORR) of 52% as determined by independent central review (ICR) using RECIST v1.1. Complete response was seen in 12 patients (18%), and 22 patients (34%) showed partial response. Among responding patients, the duration of response (DOR) ranged from 1.1 to 24.9+ months, 76% (26/34) experienced a DOR of six months or longer, and 62% (21/34) experienced a DOR of 12 months or longer by landmark analysis.
Serious adverse reactions occurred in 22% of patients receiving Zynyz. The most frequent serious adverse reactions (≥ 2% of patients) were fatigue, arrhythmia and pneumonitis. Permanent discontinuation of Zynyz due to an adverse reaction occurred in 11% of patients. The most common (≥10%) adverse reactions that occurred in patients receiving Zynyz were fatigue, musculoskeletal pain, pruritus, diarrhea, rash, pyrexia and nausea.
Continued approval of Zynyz for this indication may be contingent on verification and description of clinical benefit in confirmatory trials.