Zoryve (roflumilast) topical foam, 0.3% from Arcutis Biotherapeutics has received FDA approval for the treatment of seborrheic dermatitis in individuals 9 years of age and older. Once-daily, steroid-free Zoryve is the first drug approved for seborrheic dermatitis with a new mechanism of action in more than two decades.
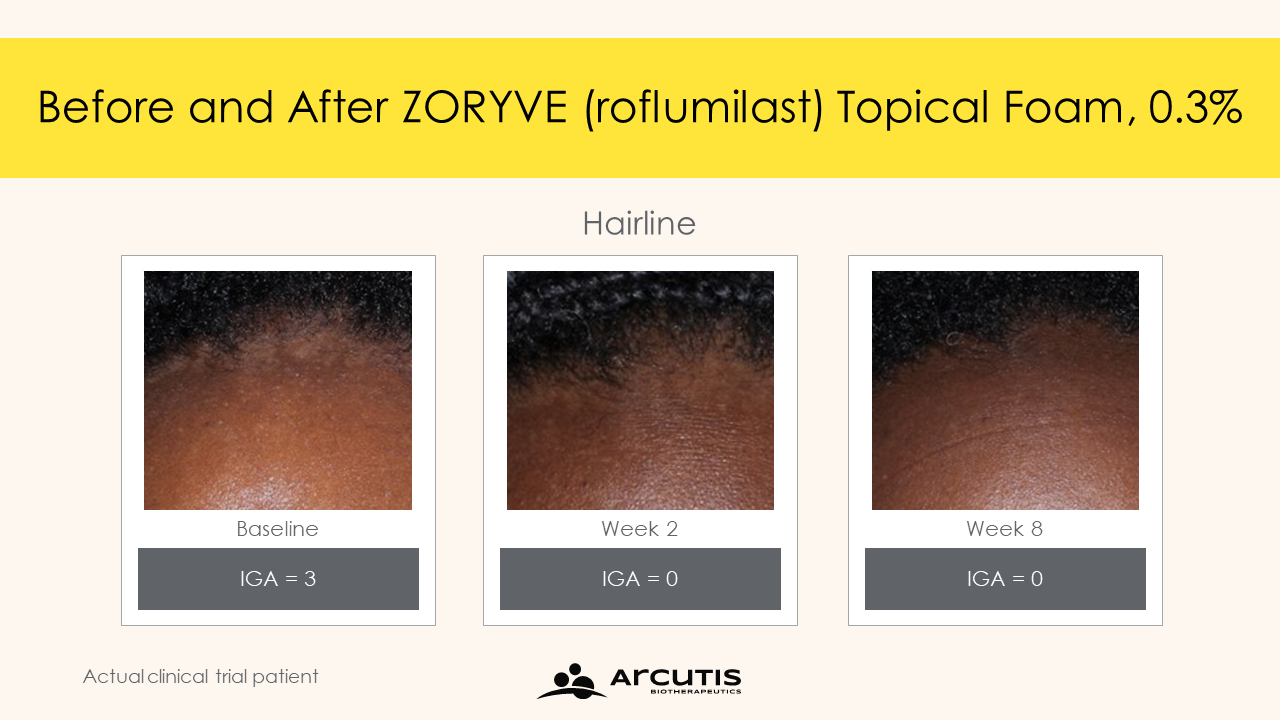
Results of the STudy of Roflumilast foam Applied Topically for the redUction of seborrheic derMatitis (STRATUM) trial show that Zoryve foam provides rapid disease clearance and significant reduction in itch, with nearly 80% of individuals achieving the primary efficacy endpoint of IGA Success and just over 50% of individuals reaching complete clearance at Week 8.
“In the STRATUM trial, ZORYVE foam provided rapid disease clearance as early as Week 2 and significant itch relief in as little as 48 hours. In addition, almost 80% of patients achieved treatment success at Week 8. While multiple factors contribute to seborrheic dermatitis, inflammation and skin barrier dysfunction play key roles. ZORYVE has been shown to effectively reduce the signs of inflammation, redness, and scaling in patients with seborrheic dermatitis, and with its unique formulation, ZORYVE foam effectively delivers the drug without disrupting the skin barrier and has been shown to be safe and tolerable. ZORYVE foam is thus ideally formulated, having the potential to become the new standard of care for seborrheic dermatitis treatment,” says Andrew Blauvelt, MD, MBA, clinical investigator at Oregon Medical Research Center, and investigator on the STRATUM trial.
Arcutis intends to make Zoryve foam widely available via key wholesaler and dermatology pharmacy channels by the end of January 2024.