As biosimilar products make their way into the field of dermatology, the latest Dermalorian™ Webinar provided a closer look at these agents and their potential role in patient care. Leigh Ann Pansch, MSN, FNP-BC, DCNP, of DOCS Dermatology in Cincinnati, OH, and T.J. Chao, MPAS, PA-C, of Atlanta North Dermatology, defined biosimilars and clarified the role of interchangeable biosimilars.
A Biologic is a product composed of proteins, nucleic acids (living) in the form of cells or tissues, that is isolated from natural sources and produced by biotechnology methods.
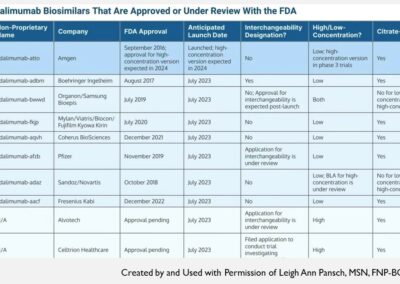
A Biosimilar is a biologic medication developed in a way where there are no clinically meaningful differences between the original product in terms of safety, purity, or potency.
As the presenters noted, in the U.S., a biosimilar may receive an “Interchangeable” designation from the FDA based on additional studies. An interchangeable biosimilar may be substituted for its reference product at a pharmacy without additional approvals from the prescriber. Individual state laws may influence the ability to switch products as well as provide requirements for reporting to the prescriber and/or patient that a switch is made.
Aside from the activity of the biologic or biosimilar treatment, the delivery system could be important, Pansch and Chao noted. Patients who have become adept at self-injection with one device may be surprised to encounter a different injection device for an interchangeable biosimilar.
Questions remain about the degree to which third party payers will encourage use of biosimilars or promote therapeutic substitutions where possible. In theory, biosimilars could provide a cost savings for some patients.
More coverage from the Dermalorian Webinar, “Same Difference: Exploring the Role for Biosimilars in Dermatology,” will be provided from DEF, including a video recording of the presentation.
Meanwhile, more information is available from:
National Psoriasis Foundation. Letter to FDA; (cited 2023 May 23). Available from: http://www.psoriasis.org/Document.Doc?id=1394.
GaBI Online – Generics and Biosimilars Initiative. FDA hearing on biosimilars: focus on characterization and clinical trials (www.gabionline.net). Mol, Belgium: Pro Pharma Communications International; (cited 2023 May 23). Available from: www.gabionline.net/Guidelines/FDA-hearing-on-biosimilars-focus-on-characterization-and-clinical-trials
National Psoriasis Foundation. Background Information on Biosimilars; (cited 2023 May 23). Available from http://www.psoriasis.org/document.doc?id=1395
For Further Reading: